
Different scientists found various elements at different times. The elements of the periodic table were not all discovered at the same time. In this article, we have explained the periodic table in its fullness to help you understand it better than before. Periodic law has contributed to the creation of the modern periodic table. All chemists use the Periodic Law when working with chemical elements, their properties, and chemical reactions. Periodic law is considered to be among the most fundamental principles of chemistry. This law was established by Dmitri Mendeleev and Lothar Meyer in 1869. It states that when elements are organized in order of increasing atomic mass, some groups of properties recur regularly. For you to understand the periodic table comprehensively, knowing what the periodic law states are essential. The periodic table is also used in schools in chemistry lessons to help students understand the various elements, their properties, and interactions with each other.
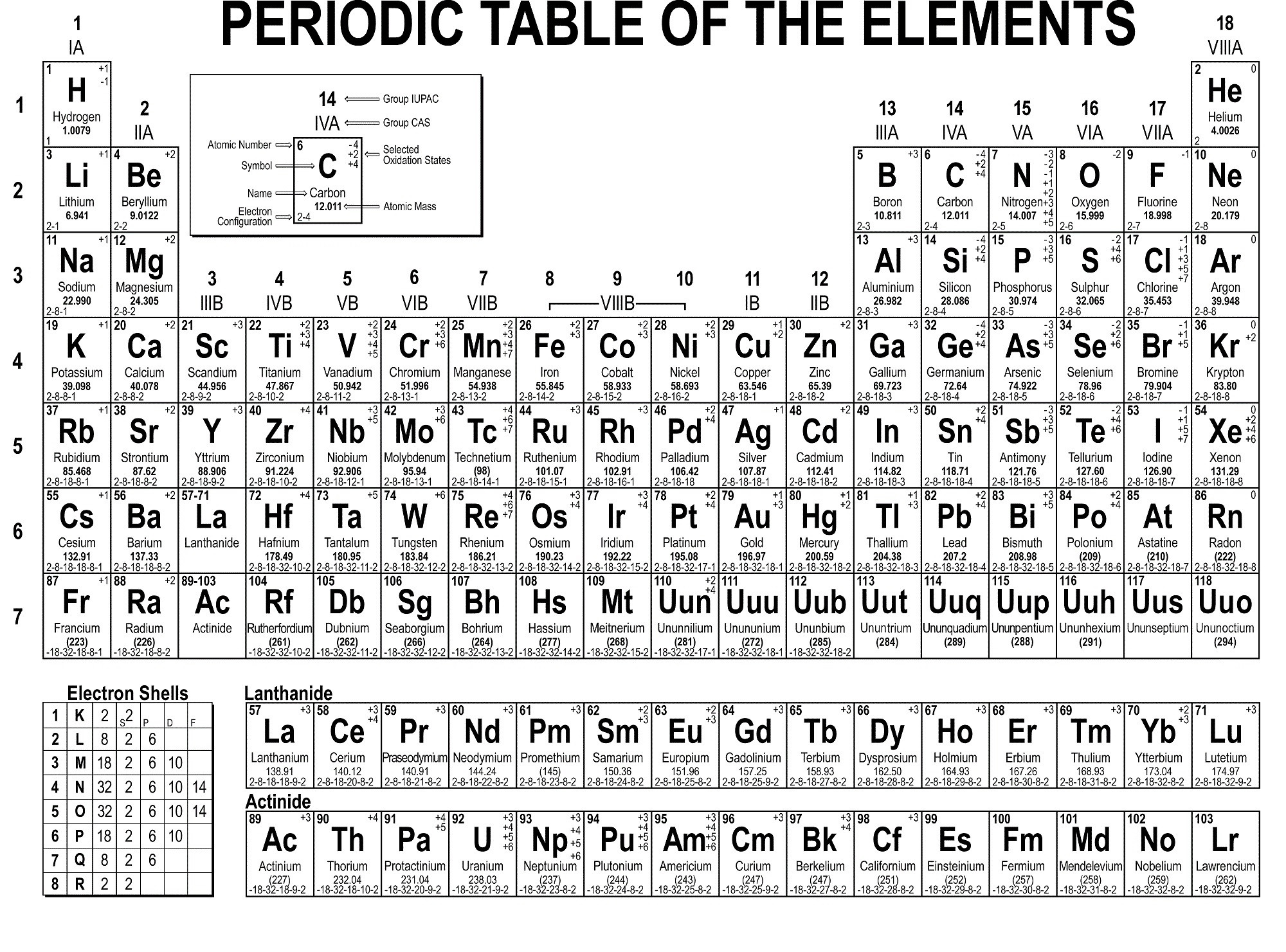
The metals appear on the left, while non-metals appear on the right side. When you go through the periodic table, you will see that metals and non-metals are on different sides of the table. It is a display of chemical elements organized by atomic number, electron configuration, and chemical properties. A periodic table (also known as the periodic table of elements) is arranged in such a manner so that you can easily distinguish the properties of various elements, such as their mass, electron number, electron structure, and their unique chemical compositions.


 0 kommentar(er)
0 kommentar(er)
